Triple Point of Water: The Temperature Where All Three Phases Coexist
Tri·ple point (/ˈtripəl point/): the temperature and pressure at which the solid, liquid, and vapor phases of a pure substance can coexist in equilibrium.
Solid. Liquid. Gas. Each of these physical states occurs naturally at different temperature and pressure ranges. At one point, the three ranges of each state coincide, creating ideal conditions for a substance to exist in all three phases. This “triple point” is different for each substance. For example, the triple point of water is reached when the temperature is 273.16 kelvins (or 0.01 °C) and the pressure is at 0.006 atmospheres, while the triple point of carbon dioxide requires the temperature of −56.6 °C and pressure of 5.11 atm. Because of the limited conditions in natural situations, the phenomenon of the triple point is very rarely, if ever, found in nature.
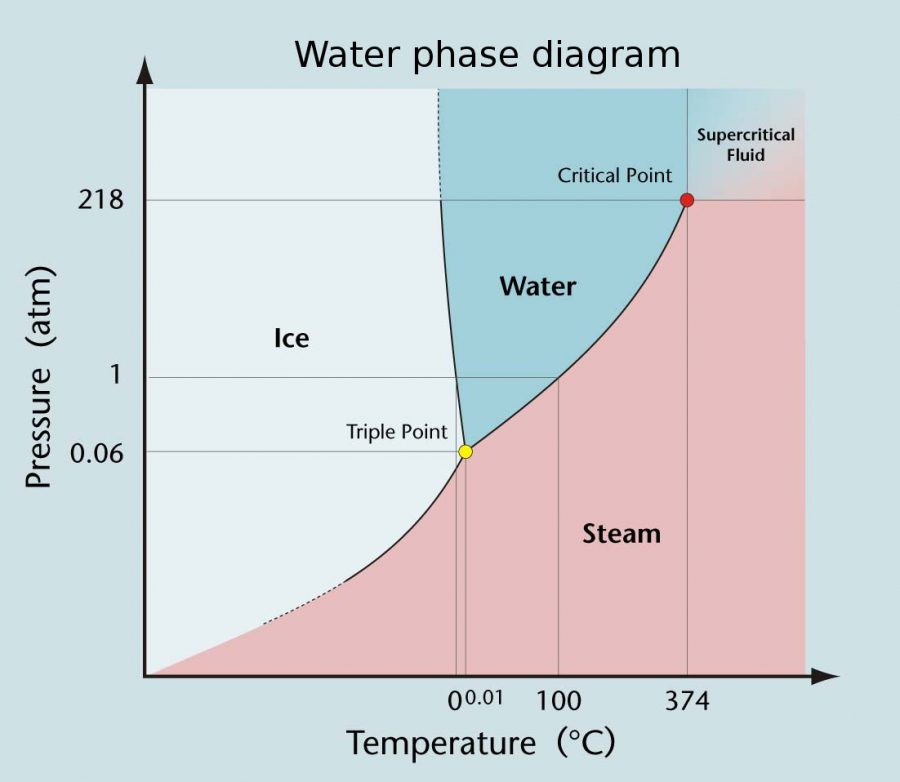
The above P-T diagram of water shows the effect of temperature and pressure on the state of water. As one moves left to right on the graph, temperature (in Celsius) increases, and as one moves bottom to top on the graph, pressure (in atmospheres) increases. The three areas on the diagram are the three different states of matter (excluding supercritical fluid) – ice, water, and steam. These phases are separated by lines called the separation lines where two different states can coexist. The line separating the area labeled “ice” and the area labeled “steam” is the sublimation line. The line between “ice” and “water” is called the melting line, and the line between “water” and “steam” is the vaporization line. The point where these three lines intersect is the triple point, where all three states can coexist at the same time because their temperature and pressure conditions are met.
The result of substances’ physical states coexisting has the result of that substance both boiling and freezing at the same time. If you were to hypothetically meet the conditions of water’s triple point, you would see both the telltale signs of ice and boiling water. Although you will rarely ever find water at its triple point in nature, you can recreate the required conditions in a vacuum in this video.
There’s no one formula that will determine the triple point of a substance because interactions between atoms of different substances are each unique with a certain set of temperature and pressure conditions. However, if an equation of state (an equation that describes a state of matter under a given set of variables, such as temperature, pressure, or volume) were given, you could potentially find the triple point of that substance. Using the EOS would allow you to find three different densities that have the same temperature and pressure. Those two values are the triple point of said substance.
Even though we can’t find water at its triple point in nature, it’s very useful in our everyday lives. Our interpretation and scale of our standard thermometer is based on the triple point of water. The reason we can’t simply use the freezing point of water to calibrate our thermometers is because the freezing point of water is dependent on the pressure. As we can see on the P-T diagram, the freezing point temperature on the melting line can vary depending on what the pressure is. However, the triple point of water is always at .01 °C, no matter what the pressure is. This lack of variability makes the triple point a simple and convenient reference point to use for our thermometers. So every time you look up the temperature, you’ll be reminded of this strange phenomenon and its impact on the world.


Derek Hendricks • Aug 15, 2025 at 1:59 pm
This folds with the suspicion that 0.06atm. is a natural settling-point for the atmospheres of rocky planets with extremely low pressure .
The H2O Triple-Point exist here , and likely stabilizes the balance of the three extant phases of water in many given environments . Given that the surface pressure of Mars is typically 0.06atm. , it is apparent that the pressure beneath the surface must be above that . If elements/compounds are present which lower the melting-point of water-ice , or temperatures are somewhat elevated , then liquid water could indeed be present .
This immediately engenders the possibility that Mars , Trappist-1d , and similar planets are inhabited by bacterial extremophiles ; life-forms which can live in environs extremely hostile to life as we know it .
Given the likely robust H2O replenishment regime for planets around red-dwarf stars , and the large number of rocky-planets within their habitable-zones , it is very likely that near-future astronomers will discover an abundance of worlds falling into the above category .
Addendum – There are a large number of planetary and stellar-system parameters and conditions which should determine which exoplanets fall into the above category , and which possess either an abundance or a dearth of volatiles and atmospheric gases .